Product Name
SARS-CoV-2 Antigen Detection Kit (NS)
Model and Specification
|
Model |
Specification |
|
LD- SC2AgO1-T01 |
1 test / kit |
|
LD- SC2AgO1-T05 |
5 tests / kit |
|
LD- SC2AgO1-T25 |
25 tests / kit |
Intended Use
This kit only be used for professional detection under supervision. SARS-CoV-2 Antigen Detection Kit is intended for professional use only. The kit is used for qualitative detection of SARS-CoV-2 antigen in human nasal cavity, nasopharynx and pharynx samples in vitro, based on the principle of latex immunochromatography- sandwich method. It is intended to be used as an aid in the diagnosis of people suspected of being infected with COVID-19.
COVID-19 is a new acute respiratory infectious disease and has become a major public health event in the world. The main sources of infection are patients with SARS-CoV-2 infections and asymptomatic infections, which are contagious during the incubation period and are highly contagious within 5 days of onset. Fever, dry cough, fatigue as the main clinical manifestations. Some patients have first symptoms such as loss of smell, loss of taste, etc., and a few patients have symptoms such as nasal congestion, runny nose, sore throat, conjunctivitis, myocardial pain and diarrhea.
Test Principle
This kit is based on the principle of latex immunochromatography - sandwich method to detect the antigens in the sample.
When the sample contains SARS-CoV-2 antigens, the red latex microspheres marked with SARS-CoV-2 antibodies on the sample pad combine with SARS-CoV-2 antigens to form an immune complex. When the immune complex moves to the T line on the nitrocellulose membrane, it combines with SARS-CoV-2 antibody again to form a red band. If the sample does not contain SARS-CoV-2 antigen, the above reaction will not occur and no red band will be generated at the T line. At any time of detection, the red latex microspheres labeled with chicken IgY will combine with the anti-chicken IgY antibody on the C line to form a red band.
Reagents and Materials Provided:
|
Reagents and Materials |
Quantity |
Quantity |
Quantity |
Description |
|
Detection card |
1 piece |
5 pieces |
25 pieces |
Foil pouched test device, |
|
1 piece |
5 pieces |
25 pieces |
For sampling |
|
|
Lysis buffer |
450μL per dropper, |
450μL per dropper, |
450μL per dropper, |
For sample dilution. |
|
Tube holder |
1 piece |
5 pieces |
25 pieces |
Instructions for use |
Materials Required but not Provided
Timer, Personal protective equipment: such as protective gloves, medical mask, goggles and lab coat. Biohazard container.
Storage and Stability
Store at temperature of 4-30°C(39.2-86°F) in a dry shady place. Avoid direct sunlight. Do not freeze the kit or its components, 18 months of shelf life (Production date to expiration date).
This product must be used in 1h at room temperature 20 to 30℃ and relative humidity between 30% and 50% after opening the inner package.
Sample Requirements
1. To avoid contamination, wear protective gloves while handling specimens when performing this test, wash hands thoroughly afterwards.
2. Collect samples as soon as possible within 7 days of symptom onset.
3. When stored in a refrigerator, all kit components must be brought to room temperature (15-30 °C) for a minimum of 30 minutes prior to performing the test. Do not open the pouch before components come to room temperature.
4. This kit is only used to detect samples collected from nasal swabs or pharyngeal swabs. Extracted specimens are stable for 4 hours in extraction buffer at room temperature.
5. It is not intended for testing other liquid sample such as nasal wash, aspirate samples or samples in viral transport media as results can be compromised by over dilution.
6. Excess blood or mucus on the swab specimen may interfere with test performance and may yield a false-positive result, avoid touching any bleeding areas when collecting specimens.
Sampling (Nasal swab sample):
|
|
|
|
|
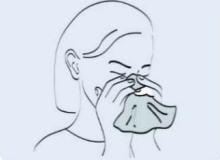
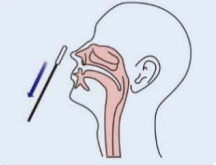
Step 1: Gently clean the nasal cavity and throw the tissue away in a closed bin. If the detection is for a child, help him to clean his nasal cavity. |
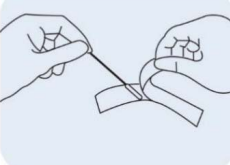
Step 2: Remove a sampling swab from the pouch. |
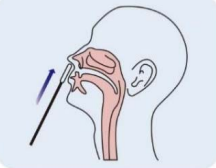
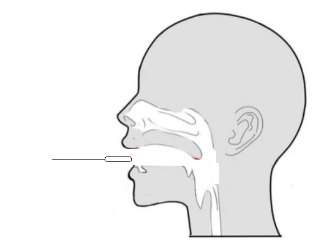
Step 3: Insert the swab into the nostril to 1 inch from the edge of the nostril or into the nasopharynx. |
|
|
|
|
|
Step 4(Nasal cavity): Slowly roll the swab 5 times over the surface of the nostril. Using the same swab, repeat this collection process in the other nostril. Take approximately 15 seconds to collect the specimen. |
Step 4(Nasopharynx): Keep insert until resistance is encountered. Slowly roll the swab for 5 times over the surface of the posterior nasopharynx. |
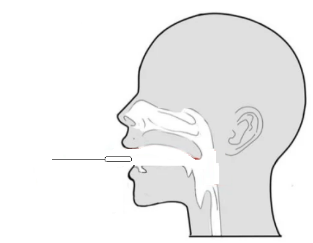
Step 5: Slowly remove the swab from the nostril while rotating it. |
Sampling (Pharynx swab sample):
|
|
|
|
|
|
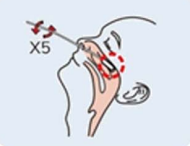
Step 1: Remove a nasal swab from the pouch. |
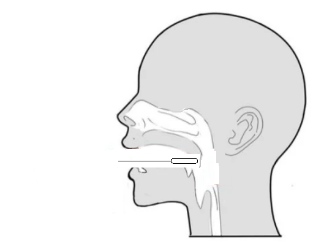
Step 2: Open your mouth and place the swab on the posterior pharyngeal wall. |
Step 3: Repeatedly wipe the posterior pharyngeal wall and lateral wall for 3 ~ 5 times to collect mucosal cells. |
Step 4: Remove the swab from the mouth to avoid touching the tongue, oral mucosa and saliva. |
Sample processing:
|
|
|
|
|
|
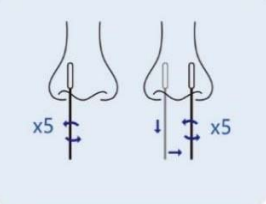
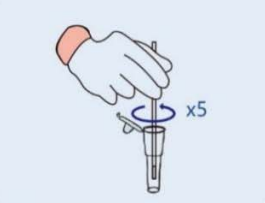
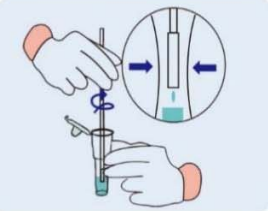
Step 1: Remove the cover of the lysis buffer tube,place the swab into the tube. Rotate the swab vigorously at least 5 times and 15 seconds. |
Step 2: Removing the swab from the tube, pinch the outer wall of the tube with your fingers to release the liquid at the end of the swab. |
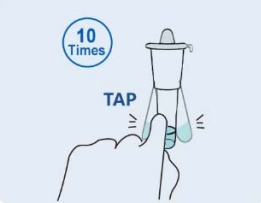
Step 3: Cover the lysis buffer tube with the cover, Mix the tube by gently tapping 10 times. Let stand for 1 minute. |
Step 4: Gently lower the top of the tube cover before use. |
Detection Procedure
Please read the instruction manual carefully before testing.
|
|
|
|
|
|
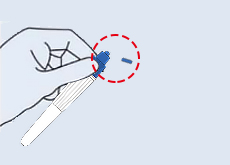
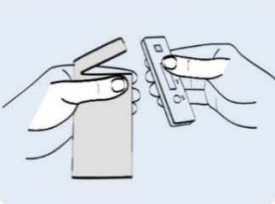
Step 1: Take the test strip out of the sealed packaging and place it onto the cleaned flat surface. Once opened, start the test within 30 minutes. |
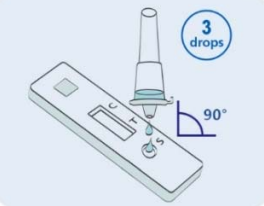
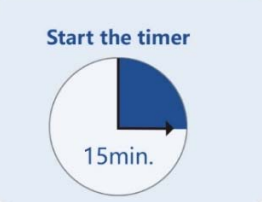
Step 2: Add three Drops (or 50-80μL) of processed specimen vertically into the sample well, and then let stand for 15 minutes. |
Step 3: Read and interpret the test result at 15 minutes. The test result should not be read and interpreted after 30 minutes. |
Safely throw away the waste into biohazard container. |
Interpretation of Results
|
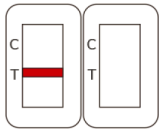
Positive |
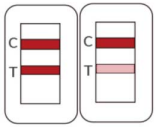 |
Two colored lines appear. One red-colored line next to “C” and one red-colored line next to “T” indicate COVID-19 positive result. |
The color intensity in the test region will vary depending on the amount of SARS-CoV-2 nucleocapsid protein antigen present in the sample. Any faint colored line(s) in the test region(s) should be considered as positive. |
|
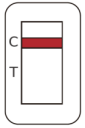
Negative |
|
One red-colored line only next to “C” indicates a negative result. |
Negative results don't preclude SARS-CoV-2 infection and they cannot be used as the sole basis for treatment or other management decisions. Should be confirmed with a molecular assay. Negative results must be combined with clinical observations, patient history, and epidemiological information. |
|
Invalid |
|
If the red-colored line in the control region “C” is not visible, the result is invalid. |
Re-run the test one time using the remaining specimen in the extraction vial if an invalid result is obtained during initial testing. |
Test Limitations
1. The test results of this product are for diagnostic aid only and cannot be used as the sole basis for confirming or excluding the diagnosis. To achieve diagnostic purposes, the results should always be assessed in combination with clinical examination, medical history, and other laboratory data.
2. This product is only used for the professional qualitative detection of SARS-CoV-2 antigen from nasal cavity, nasopharynx or pharynx swab, other specimen types may lead to incorrect results and must not be used, not for quantitative detection which provide information on the viral concentration present in the specimen.
3. This product is only for the initial screening test. The disease diagnosis should be made in conjunction with clinical observations, patient history, epidemiological information, and other laboratory evidence.
4. Subject to the limitations of the assay methodology, the questionable results should be verified with other test methodologies.
5. Failure to follow the instructions for test procedure and interpretation of test results may adversely affect test performance and/or produce invalid results.
6. Positive test results do not rule out co-infections with other pathogens.
7. False-negative test results may occur if a specimen is improperly collected transported, or handled.
8. False-negative test results may occur if significant virus mutation, improperly treated antigen degeneration.
9. False-negative test results may occur if the level of an antigen in a sample is below the detection limit of the test. Test performance depends on the amount of virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same sample.
10. False negative results are more likely after eight days or longer of symptoms.
11. False positive results may occur if cross-contamination and other unverified factors.
12. Negative test results are not rule out diseases caused by other non-SARS-CoV-2 viral or bacterial infections.
13. If the differentiation of specific SARS viruses and strains is needed, additional testing, in consultation with state or local public health departments, is required.
14. The performance of this test has not yet been clinically validated for use in patients without signs and symptoms of respiratory infection, or for serial screening applications when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests, and performance may differ in these populations. A study to support use for serial testing will be completed.
Warnings and Precautions
1. For in vitro diagnostic use only.
2. The instructions must be strictly followed by a trained healthcare professional to achieve accurate results. All users have to read the instruction prior to performing a test.
3. Do not use the kit if opened, damaged, or expired, keep test card sealed in its foil pouch before use.
4. To avoid cross-contamination, do not reuse the specimen collection swab.
5. Do not mix or interchange different specimens.
6. Do not dilute the collected swab with any solution except for the provided extraction buffer.
7. Inadequate or inappropriate sample collection, storage, and transport may cause false test results.
8. Samples with invalid results must be retested.
9. Clean up spills thoroughly using an appropriate disinfectant.
10. Do not store the test kit in direct sunlight.
11. Contaminated work clothing should not be allowed out of the workplace.
12. Dispose of contents/container to an approved waste disposal plant.
13. All human material should be considered potentially infectious.
Clinical Performance
In this clinical study, 510 participants were tested with SARS-CoV-2 Test Kit and RT-PCR. Two bilateral anterior nasal swab samples were collected from each participant. An RT-PCR assay for detecting SARS-CoV-2 from one swab is utilized as the comparator method for the study. All the anterior nasal swab specimens were confirmed as positive or negative and validated with Ct value by the RT-PCR. The performance of the SARS-CoV-2 Ag Test Kit as compared to the RT-PCR comparator method are presented in the table below:
|
SARS-Cov-2 Antigen Detection Kit |
Comparator(RT-PCR) |
||
|
Positive |
Negative |
Total |
|
|
Positive |
162 |
2 |
166 |
|
Negative |
3 |
343 |
346 |
|
Total |
165 |
345 |
510 |
|
Sensitivity |
162/165, 98.18% (95%CI, 94.79%~99.38%) |
||
|
Specificity |
343/345, 99.42% (95%CI, 97.91%~99.84%) |
||
454 participants were tested with SARS-CoV-2 Test Kit and RT-PCR. Two nasopharyngeal swab samples were collected from each participant. An RT-PCR assay for detecting SARS-CoV-2 from one swab is utilized as the comparator method for the study. All the nasopharyngeal swab specimens were confirmed as positive or negative and validated with Ct value by the RT-PCR. The performance of the SARS-CoV-2 Ag Test Kit as compared to the RT-PCR comparator method are presented in the table below:
|
SARS-Cov-2 Antigen Detection Kit |
Comparator(RT-PCR) |
||
|
Positive |
Negative |
Total |
|
|
Positive |
122 |
2 |
124 |
|
Negative |
4 |
326 |
330 |
|
Total |
126 |
328 |
454 |
|
Sensitivity |
122/126, 96.83% (95%CI, 92.12%~97.80%) |
||
|
Specificity |
326/328, 99.39% (95%CI, 97.80%~99.83%) |
||
481 participants were tested with SARS-CoV-2 Test Kit and RT-PCR. Two pharynx swab samples were collected from each participant. An RT-PCR assay for detecting SARS-CoV-2 from one swab is utilized as the comparator method for the study. All the nasopharyngeal swab specimens were confirmed as positive or negative and validated with Ct value by the RT-PCR. The performance of the SARS-CoV-2 Ag Test Kit as compared to the RT-PCR comparator method are presented in the table below:
|
SARS-Cov-2 Antigen Detection Kit |
Comparator(RT-PCR) |
||
|
Positive |
Negative |
Total |
|
|
Positive |
135 |
3 |
138 |
|
Negative |
5 |
338 |
343 |
|
Total |
140 |
341 |
481 |
|
Sensitivity |
135/140, 96.43% (95%CI, 91.91%~98.47%) |
||
|
Specificity |
338/341, 99.12% (95%CI, 97.45%~99.70%) |
||
Limit of Detection (Analytical Sensitivity)
A preliminary limit of detection was established using heat-inactivated SARS-CoV-2 isolate USA-WA1/2020. The strain was spiked into the pooled human nasal swab matrix obtained from multiple healthy volunteers eluted in VTM and confirmed as SARS-CoV-2 negative by RT-PCR to prepare positive samples. The estimated LoD found from the initial five-fold serial dilution test was confirmed by testing 20 replicates. The LoD was confirmed as the lowest concentration of SARS-CoV-2 that was detected ≥95% of the time. The SARS-CoV-2 Antigen Test Kit was confirmed to be 2.0×102 TCID50/mL.
|
LOD Concentration TCID50/mL |
Number of Positive/Total |
% Detected |
|
2.0×102 |
20/20 |
100% |
Analytical Specificity: Cross Reactivity (Exclusivity) and Microbial Interference
Cross-Reactivity and Microbial Interference studies were conducted to determine if other respiratory pathogens that could be present in a nasopharyngeal sample could cause a false-positive test result, or interfere with a true positive result. A panel viruses bacteria fungus listed below, and pooled human nasopharyngeal swab was evaluated in this study. No cross-reactivity or interference was seen with the following microorganisms when tested at the concentrations listed in the table below with the exception of SARS-CoV, which resulted in positive test results due to the high homology between SARS-CoV and SARS-CoV-2 nucleocapsid proteins.
|
Potential Cross Reactant |
Source/Strain/ID No. |
Concentration Tested |
|
|
Virus |
Adenovirus 1 |
ATCC VR-1 |
1.43 ×105 TCID50/mL |
|
Human metapneumovirus(hMPV) |
Zeptometrix 0810157CF |
1.43 ×105 TCID50/mL |
|
|
Rhinovirus |
ATCC VR-1601 |
4.45 ×105 TCID50/mL |
|
|
Enterovirus 68 |
ATCC VR-1826 |
8.0 ×105 TCID50/mL |
|
|
Human Coronavirus OC43 |
Zeptometirx 0810024CF |
1.43 ×105 TCID50/mL |
|
|
Human Coronavirus 229E |
ATCC VR-740 |
1.43 ×105 TCID50/mL |
|
|
Human Coronavirus NL63 |
BEI Resources |
1.43 ×105 TCID50/mL |
|
|
SARS-coronavirus |
MRI Urbani |
7.9 ×103 TCID50/mL |
|
|
MERS-coronavirus |
MRI EMC/2012 |
2.5 ×104 TCID50/mL |
|
|
Parainfluenza virus 1 |
ATCC VR-94 |
1.43 ×105 TCID50/mL |
|
|
Parainfluenza virus 2 |
ATCC VR-92 |
1.43 ×105 TCID50/mL |
|
|
Parainfluenza virus 3 |
ATCC VR-93 |
1.43 ×105 TCID50/mL |
|
|
Parainfluenza virus 4ba |
Zeptometrix 0810060BCF |
8.5 ×104 TCID50/mL |
|
|
Parainfluenza virus 4bb |
ATCC VR-1377 |
8.0 ×104 TCID50/mL |
|
|
Influenza A |
ATCC VR-1894 |
1.43 ×105 CEID50/mL |
|
|
Influenza B |
ATCC VR-1931 |
1.43 ×105 TCID50/mL |
|
|
Respiratory syncytial virus |
ATCC VR-26 |
4.0 ×106 PFU/mL |
|
|
Bacteria |
Bordetella pertussis |
ATCC 9797 |
1.0 ×106 cfu/mL |
|
Chlamydia pneumoniae |
ATCC VR-2282 |
1.0 ×106 IFU/mL |
|
|
Haemophilus influenzae |
ATCC 49247 |
1.0 ×107 cfu/mL |
|
|
Legionella pneumoniae |
Zeptometrix 801645 |
1.0 ×106 cfu/mL |
|
|
Strepotococcus pneumoniae |
ATCC 49319 |
4.48 ×105 cfu/mL |
|
|
Streptococcus pyogenes |
ATCC 19615 |
1.0 ×106 cfu/mL |
|
|
Mycoplasma pneumoniae |
ATCC 15531-TTR |
1.0 ×105 cfu/mL |
|
|
Staphylococcus aureus |
ATCC 12600 |
1.0 ×106 cfu/mL |
|
|
Staphylococcus epidermidis |
ATCC 14990 |
1.0 ×106 cfu/mL |
|
|
Mycobacterium tuberculosis |
Zeptometrix 801660 |
1.0 ×106 cfu/mL |
|
|
Fungi |
Candida albicans |
ATCC 14503 |
5.0 ×106 cfu/mL |
|
Pneumocystis carinii |
ATCC PRA-159 |
1.0 ×106 nuclei/mL |
|
|
P. jiroveci-S. cerevisiae recombinant |
Zeptometrix 801698 |
1.0 ×106 cfu/mL |
|
|
|
Pooled Human nasopharyngeal swab |
Lee Biosolutions 991-26 |
N/A |
a Used for Exclusivity Testing b
Used for Microbial Interference
Cross reactivity in samples containing HKU1 coronavirus could not be conclusively ruled out through in silico comparison of the HKU1 and the SARS-CoV-2 nucleocapsid protein amino acid sequence. Additionally, the SARS-CoV-2 Nucleocapsid protein sequence was BLAST aligned on the NIH NCBI database to the entire set of proteins encoded by P. jirovecii. No significant identity was found as a result of this search and thus no interference is expected with the SARSCoV-2 Antigen Test Kit, however, cross-reactivity cannot be ruled out.
Endogenous Interfering Substances Effect
A study was conducted to determine if any substances, naturally present in respiratory specimens or that may be artificially introduced into the nasal cavity listed in the table interfere in the performance of the SARS-CoV-2 Antigen Test Kit. In addition to the materials that are found in the nasal cavity, substances that are commonly found on the hands were also tested. Test performance was evaluated in the absence and presences of SARS-CoV-2 (3x LoD). None of the substances listed in the tables below interfered with the performance of the SARS-CoV-2 Antigen Test Kit.
|
Potential Interfering Substances |
Active Ingredient |
Concentration |
|
Human Whole Blood (EDTA tube) |
American Blood Bank |
4% |
|
Mucin (porcin stomach, type II) |
Sigma M2378 |
0.5% |
|
Chloraseptic (Menthol/Benzocaine) |
Chloraseptic Max |
1.5 mg/mL |
|
Naso GEL (NeilMed) |
NeilMed |
5% v/v |
|
Nasal Drops (Phenylephrine) |
CVS Health |
15% v/v |
|
Nasal Spray (Oxymetazoline) |
CVS Health |
15% v/v |
|
NasalSpray (Cromolyn) |
Nasal Crom |
15% v/v |
|
Zicam |
Zicam |
5% v/v |
|
Homeopathic (Alkalol) |
Alkalol |
10% v/v |
|
Sore Throat Phenol Spray |
Chloraseptic |
15% v/v |
|
Tobramycin |
Sigma T4014 |
4 µg/mL |
|
Mupirocin |
Sigma M7694 |
10 mg/mL |
|
Tamiflu (Oseltamivir Phosphate) |
Acros 461170050 |
5 mg/mL |
|
Fluticasone Propionate |
CVS Health |
5% v/v |
|
Biotin |
Sigma B4501 |
3.5 µg/mL |
|
Disinfectant Wipes (Alkyl (C14 (50%), C12 (40%), |
Lysol |
1 wipe |
|
Hand Sanitizer Gel (70% ethyl alcohol) |
CVS |
1.038 g |
|
Bleach Wipes (0.525% bleach) |
Hype-wipe |
1 wipe |
|
Hand Lotion |
Corn Huskers |
0.991 g |
|
Hand Lotion with Aloe |
Gold Bond Healing |
1.013 g |
|
Hand Lotion with Coconut Oil, Cocoa Butter, and African Shea Butter |
Gold Bond Ultimate Healing |
1.067 g |
|
Hand Soap |
Softsoap Fresh Breeze |
1.055 g |
High-dose Hook Effect
No high dose hook effect was observed when tested with up to a concentration of 0.5×106.0 TCID50/mL of heat inactivated SARS-CoV-2 virus.
Description of Symbols
|
|
Manufacturer |
|
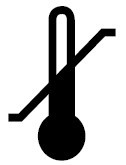
Temperature limit |
|
|
In vitro diagnostic medical device |
|
Batch code |
|
|
Catalogue number |
|
Consult instructions for use |
|
|
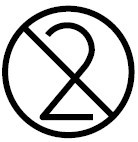
Do not re-use |
|
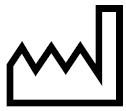
Date of manufacture |
|
|
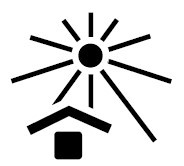
Keep away from sunlight |
|
Caution |
|
|
Biological risks |
|
Keep dry |
|
|
Do not use if package is damaged |
|
Contains sufficient for <x> tests |
|
|
Use-by date |
|
Do not resterilize |
Basic information of the Manufacturer
Name: Nanjing Leading Medical Technology Co., Ltd.
Address: Unit 408-410, Block C6, 9 Xian Lin Da Xue Wei Di Rd, Xian Lin Community, Qi Xia District, Nanjing City, Jiangsu Province, China
Tel: +86 (25) 8967 4295
E-mail: postmaster@njliding.com
Website: http://www.njliding.com/
European Representative:
Name: Share Info GmbH
Add: Heerdter Lohweg 83, 40549 Düsseldorf
Tel: 0049 1795 6665 08
Dimdi Code: DE/0000049303
Version 1.0
Preparation date: 08/10/2021












 400-0066-825
400-0066-825

 在线留言
在线留言 